electron affinity graph|Electron affinity (data page) : Clark 119 rows — Ago 11, 2023 — Electron affinity is the amount of energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a . More Fun Survey Questions with AhaSlides. It is never so easy to design a fun and lively survey for your future projects and virtual meetings whether your target is either kids or adults, school students or .
electron affinity graph,119 rows — Ago 11, 2023 — Electron affinity is the amount of energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a .Ene 30, 2023 — Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative .
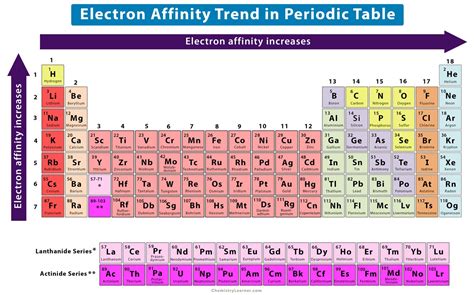
Electron Affinity Chart (Labeled Periodic table + List)
Electron Affinity: Definition, Chart & Trend in Periodic Table

Electron Affinity Trend and Definition - Science Notes and Projects
Electron Affinity Chart (Labeled Periodic table + List)For example, the first electron affinity of oxygen is −141 kJ/mol, but the second electron affinity is +744 kJ/mol: \[O_{(g)} + e^- \rightarrow O^-_{(g)} \;\;\; EA_1=-141 \;kJ/mol .Electron affinity (data page) The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e → X (g) + energyThis differs by sign from the energy change of electron capture ionization. The electron affinity is positive when energy is released on electron capture.119 rows — Electron affinity can be defined in two equivalent ways. First, as the energy .What is Electron Affinity. The electron affinity is defined as the amount of energy released per mole when an electron is added to a neutral atom. It is the opposite of ionization energy [1-4]. How to Find Electron Affinity. The .electron affinity graph Electron affinity (data page) Set 20, 2022 — The energy change that occurs when a neutral atom gains an electron is called its electron affinity. When energy is released in a chemical reaction or process, that energy is expressed as a negative .
Electron affinity refers to the energy released when an additional electron is attached to a neutral atom to form a singly charged negative ion. Alternatively, it can also be defined as the energy required to detach an .Ago 31, 2022 — Electron affinity is a measure of how readily a neutral atom gains an electron. Electron affinity (Eea) is the energy change when an electron is added to a neutral atom in the gas phase. In simple terms, it .
The electron affinity of an atom is the energy released when an electron is added to a neutral atom in the gas state to form a negative ion, per mole of atoms. Electron .Dis 8, 2013 — Watch a video explaining how electron affinity varies across the periodic table and why it matters for chemistry. Khan Academy offers free, high-quality education for anyone, anywhere.A large amount of energy is released when the atom transforms into an ion. Hence, the electron affinity values are higher. Therefore, the electron affinity increases from left to right across a period, as shown in the .electron affinity graphWhen atoms gain electrons they become negative ions or anions; Electron affinity (EA) can be thought of as the opposite process of ionisation energy and is defined as The amount of energy released when one mole of electrons is gained by one mole of atoms of an element in the gaseous state to form one mole of gaseous ions; Electron affinities .
The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right .May 9, 2021 — Electron Affinity is the amount of energy RELEASED when you add an electron to a particle in the gas phase. So the general equation is:A + electron = A(-) + .Figure 4.4.1 graphs the relationship between the first ionization energy and the atomic number of several elements. . Electron affinity (the energy associated with forming an anion) is more favorable (exothermic) when electrons are placed into lower energy orbitals, closer to the nucleus. Therefore, electron affinity becomes increasingly .Definition: Electron Affinity defined as removal of an electron. Electron affinity can be defined as the energy required when an electron is removed from a gaseous anion. The reaction as shown in equation \(\ref{EA1}\) is endothermic (positive \(\Delta U\)) for elements except noble gases and alkaline earth metals. Under this definition, the more positive .Ago 28, 2023 — Definition of Electron Affinity. Electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gaseous atom. The more negative the electron affinity value, the higher the electron affinity and the more easily an electron is added to an atom. Electron affinity can be .
The first ionization energy for sodium is one and one-half times larger than the electron affinity for chlorine. Na: 1st IE = 495.8 kJ/mol. Cl: EA = 328.8 kJ/mol. Thus, it takes more energy to remove an electron from a neutral sodium atom than is given off when the electron is picked up by a neutral chlorine atom.The electron affinity of an element is the energy released when an electron is added to a neutral atom of that element in the gas state, to form a singly negative ion (it is also called the first electron affinity). A positive electron affinity indicates that the process releases energy (it is exothermic), lowering the energy of the system. .Ene 16, 2023 — Electron Affinities. Electron affinity, often abbreviated as EA, is the energy released when an electron is added to a valence shell of the atom. F(g) + e - -> F-(g) EA = -328 kJ/mol [When an electron is .Ago 13, 2021 — The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron affinities (the highest affinity for an added electron) are those with the smallest size and highest ionization energies and are located in the upper right .Set 27, 2022 — The opposite of IE is described by electron affinity (EA), which is the energy change when a gas-phase atom accepts an electron: \[A(g)+e^{-}\rightarrow A^{-}(g)\; \; \; \; \; \Delta H\equiv EA \nonumber \]. EA is also usually expressed in kJ/mol. EA also demonstrates some periodic trends, although they are less obvious than the other .Peb 9, 2024 — The electron affinity depends upon the nuclear charge and atomic size. Electron affinity is directly proportional to the nuclear charge on the atom and inversely proportional to its atomic size. Hence we can conclude: Electron affinity increases across the periodic table from left to right due to nuclear charge increases.Electron affinity (the energy associated with forming an anion) is more favorable (exothermic) when electrons are placed into lower energy orbitals, closer to the nucleus. Therefore, electron affinity becomes increasingly negative as we move left to right across the periodic table and decreases as we move down a group. For both IE and electron .
Okt 7, 2022 — where E is the photon energy, E EA is the electron affinity, and l is the angular momentum quantum number of the outgoing electron 30.In the case of oxygen, the 2p electron can be emitted as an s .

Ago 2, 2021 — Figure 3.3.8 A Bar Graph of Electronegativity for Atoms in the First Six Rows of the Periodic Table. Linus Pauling (1901–1994) . The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the most negative electron affinities (the highest .
electron affinity graph|Electron affinity (data page)
PH0 · Lesson Explainer: Electron Affinity
PH1 · Electron affinity (data page)
PH2 · Electron affinity
PH3 · Electron Affinity: Definition, Chart & Trend in Periodic
PH4 · Electron Affinity Trend and Definition
PH5 · Electron Affinity Chart (Labeled Periodic table + List)
PH6 · Electron Affinity
PH7 · 7.5: Electron Affinities
PH8 · 6.19: Periodic Trends